Abstract
In sickle cell disease (SCD) cerebrovascular complications, including white matter injury and cognitive dysfunction, contribute to significant morbidity and mortality, however, the link between white matter injury and cognitive deficits has not been fully elucidated. Ischemia is a significant risk factor for brain injury in SCD but the processes that mediate ischemic-related brain damage are not known. Astrocytes residing in the white matter are characterized by the expression of the intermediate filament protein glial fibrillary acidic protein (GFAP) that is upregulated during ischemic brain injury. Several lines of evidence suggest that astrocyte activation is linked to neuronal injury. Thus, we set out to explore whether white matter injury is linked to cognitive dysfunction in sickle cell mice and associated with astrocyte activation.
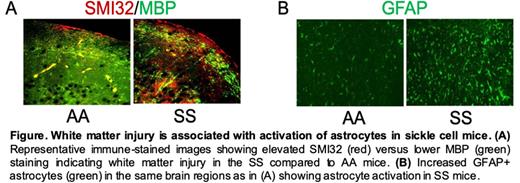
First, we found that the homozygous Townes sickle cell (SS) mice that express human sickle hemoglobin (HbSS) phenocopy humans with SCD by displaying increased white matter injury and microstructural cerebral tissue damage compared to control Townes (AA) littermate mice possessing normal human hemoglobin (HbAA). We performed T2-weighted MRI, diffusion tensor imaging (DTI), and arterial-spin labeling (ASL) MRI for quantitative perfusion measurements in SS and AA mice. Analysis of the DTI data showed that fractional anisotropy (FA), an axonal tissue damage indicator based on water diffusion directionality, was substantially lower in SS mice than in AA mice (p<0.05, n=6), indicating loss of white matter integrity. We then confirmed white matter injury histologically by measuring the expression of non-phosphorylated neurofilament H (SMI32) in relation to myelin basic protein (MBP), and found an increased SMI32/MBP ratio - a marker of white matter injury - in SS mice as compared to AA controls (p<0.05, n=4-6). The accumulation of neurofilament light chain (NFL) protein was also significantly elevated in SS mice plasma and brain, indicating disruption of neuro-axonal integrity. Astrocytes interconnect cerebral microvasculature and neurons and maintain neuronal cell integrity. Increased NFL accrual is associated with activation of astrocytes, loss of neuronal plasticity and impaired memory function in several diseases including Alzheimer's, Parkinson's and multiple sclerosis. We hypothesized that white matter injury in SS mice would be associated with reduced neurocognitive responses and activation of astrocytes. We performed the novel object recognition (NOR) and Y-maze cognitive tests in SS and AA mice. Quantitation of exploration time and discrimination index showed lower NOR ability of the SS mice compared to the AA counterpart (p<0.05, n=10). The SS mice displayed a lower percentage of spontaneous alterations in a Y-maze compared to AA mice indicating impaired memory function. As expected, based on our hypothesis, plasma NFL concentration was inversely correlated with percent exploration time showing that poor NOR ability is associated with increased plasma NFL in the SS mice. Finally, we observed that SS mice had a significantly higher number of GFAP-positive, activated astrocytes compared to AA mice.
In conclusion, we found that white matter injury is linked to compromised cognitive responses and is associated with activation of astrocytes in sickle cell mice. Better elucidation of the interplay between astrocytes and neurons during ischemic conditions in sickle cell mice may enhance our understanding of cerebrovascular complications in SCD, and provide pharmacologic targets to improve cognitive function in patients with SCD.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal